Person, Project, Place
Break-through research needs talented enthusiastic scientists, matched to well designed projects, in a supportive place. The Spillantini lab has a long history of attracting postdoctoral researchers and PhD students from around the world. Many have gone on to start their own labs and pursue independent research careers. Others have moved into industry or successful consulting roles. You can find the profiles of current members below.
Want to join?
Do you think you have a novel research idea? Do you have the drive to win funding? Do you want to perform world-class research at the University of Cambridge? Then please contact us to book an appointment. Call +44 (0)1223 331160 or email mgs11@cam.ac.uk.
Postdoctoral Research Associates
|
|
Dr. Aviva Tolkovsky (amt1004@cam.ac.uk)
Nerve cells are complicated highly branched cells that are essential for our brains and our bodies. Without nerve cells we cannot think, feel, walk, pump blood or digest our food. The protein tau is a nerve cell protein that enables neurons to distribute essential proteins to distant parts of the cell in the brain, spinal cord, and in neurons that mediate sensation and control the actions of our internal organs. In Alzheimer's disease, tau ceases to function normally; it becomes misfolded and eventually forms neurofibrillary tangles (NFT) that are thought to be critical for neurodegeneration in the disease.Quite a lot is known about pathological changes that occur in tau, but one fundamental problem is still unanswered: what form of tau is truly pathological and how tau causes the nerve cells to die? These problems are difficult to solve by looking at the nervous system in post-mortem human brains because it is difficult to catch a dying nerve cell as dead nerve cells are rapidly cleared by neighbouring cells. For this reason, mouse models of tauopathy have been created where several features of the disease are replicated. However, even in mice, the dying cells in the brain are not easily accessible. To reduce animal use and still be able to investigate how tau kills cells, we have turned to a cell culture system of nerve cells from the sensory nervous system. We now know that cell death is not necessarily a random process. It can involve the orderly demolition of the cell. We develop models to determine the pathways by which tau causes nerve cell pathology and death. We also wish to explore whether we can keep the sick nerve cells alive using drugs that are being developed for eventual use in humans.
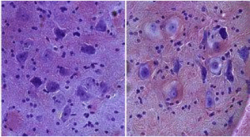
Image: Brain sections from two transgenic littermates expressing mutated tau protein stained with Cresyl Violet. Left panel shows healthy neurons in a mouse before disease onset, right hand panel shows swollen balloon neurons close to death from a symptomatic mouse |
|
Mehtap Bacioglu (mb2262@cam.ac.uk) The deposition of aggregated proteins in the brain is a pathological hallmark of proteopathic neurodegenerative diseases, such as tau in Alzheimer's disease and frontotemporal dementia (FTDP-17T). I am interested in understanding how protein aggregation and propagation contribute to neurodegenerative diseases and exploring the potential for stem cell models to bridge the gap between animal studies and human applications. Specifically, I use induced pluripotent stem cell-derived brain organoids from affected individuals and healthy donors to model and investigate tau aggregation and related neurodegeneration. This approach aims to provide deeper insights into disease mechanisms and identify potential therapeutic targets.
|
|
|
Dr. Emre Fertan (ef417@cam.ac.uk) Mitochondrial damage and oxidative stress are common features of neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease. However, the causal and potentially bi-directional relationship between alpha-synuclein aggregation and mitochondrial dysfunction, and how this is related to mitochondrial DNA mutations has not been fully understood. The aim of my research is to study the mechanisms behind mitochondrial dysfunction in Parkinson’s disease, such as the role of mitochondria DNA mutations to improve our understanding of the relationship between alpha-synuclein aggregation and mitochondrial dysfunction.
|
|
| Dr. Gabriele Strusi (gs784@cam.ac.uk) | |
| Dr. Francesa Longhena (fl433@cam.ac.uk) | |
| Dr. Giorgio Vivacqua (giorgio83.vivacqua@gmail.com) |
PhD Students
|
|
Janine Brandes (jb2274@cam.ac.uk)
|
| |
Matthew Mason (mm2458@cam.ac.uk)
Ageing is one of the greatest risk factors for idiopathic neurodegenerative disease. Indeed, in recent years, it has been demonstrated that there is a significant degree of overlap between the cellular changes that occur in natural ageing and those that occur in neurodegenerative disease. The objective of my PhD project is to investigate this overlap, with a specific focus on the activity of glia in these contexts. To this end, I am generating cortical organoids and iPSC-derived microglia, to explore in vitro how disease-relevant stimuli and ageing-relevant stimuli might dovetail to perturb the function of microglia.
|
|
Pauline Vessiere (paav2@cam.ac.uk) Parkinson’s disease (PD) is a neurodegenerative disorder primarily known for its motor symptoms. However, PD also presents multiple pre-motor symptoms that often manifest in the nose and gut, such as loss of smell (anosmia) and gastrointestinal issues like constipation. These early indicators, appearing years before motor symptoms, highlight the role of peripheral sites in the disease's onset and progression. Due to these peripheral symptoms, α-synuclein, a protein that misfolds in PD, is believed to transfer from peripheral sites such as the nose and gut to the brain. To better understand the transfer of α-synuclein from the periphery to the brain, I am working on characterizing novel mouse models of PD created in this lab. Exploring the early events of PD will potentially offer new avenues for early diagnosis and intervention.
|
|
| Giulio Deangeli (gd456@cam.ac.uk) |
Visiting Researchers
|
Rafailia Christou (rc932@cam.ac.uk) |
Lab Technicians
|
Emma Carlson (Lab Manager) (ec596@cam.ac.uk) |
| Zhiguang "Kevin" Zheng (zz217@cam.ac.uk) |
Lab Alumni
- Dr. Jack H. Brelstaff
- Dr. Laura Calo
- Dr. Joana Domingues
- Dr. Steven Fagan
- Dr. Solène Ferreira
- Helen Henson
- Dr. Sanne Kaalund
- Dr. Aishwarya G. Nadadhur
- Dr. Clara Pérez-falo
- Dr. Katrina Räty
- Dr. Ellen Tedford